Deepmaterial
Best UV Adhesive Manufacturer
Custom Adhesives for your requirements
High Tech Industrial Adhesives
We provide industrial adhesives as well as medical grade adhesives,
our adhesives are used wherever reliable, durable, and fast connections are required.
DeepMaterial Epoxy Adhesive for Electric
Deepmaterial is reactive hot melt pressure sensitive adhesive manufacturer and supplier, manufacturing one component epoxy underfill adhesives, hot melt adhesives glue, uv curing adhesives, high refractive index optical adhesive, magnet bonding adhesives, best top waterproof structural adhesive glue for plastic to metal and glass, electronic adhesives glue for electric motor and micro motors in home appliance.
PUR Structural Adhesive

UV Moisture Dual Curing Adhesive

Two-component Epoxy Adhesive

For Electronics
Low Temperature Curing Epoxy Adhesive
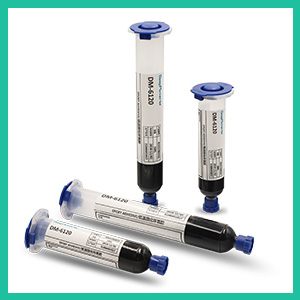
Two-component Epoxy Adhesive
Industrial Adhesive Applications
Deepmaterial provide electronic adhesives and thin-film electronic application materials products and solutions for communication terminal companies, consumer electronics companies, semiconductor packaging and testing companies, and communication equipment manufacturers, to solve the above-mentioned customers in process protection, product high-precision bonding, and electrical performance. Domestic substitution demand for protection, optical protection, etc.
Industrial Adhesive Solutions
Deepmaterial Adhesives specializes in adhesives for the plastic, glass, metal products industry. We offer a full range of products for almost every imaginable application within this market. Below you will find a number of different adhesive categories and a description of the available products within each category.

Elastic
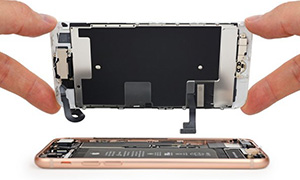
Transparent
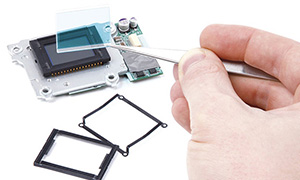
Low-viscosity

High-viscosity

Low temperature

Fast curing

Heat-cured
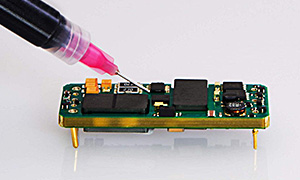
High-temperature

Fast curing
About DeepMaterial
Best Adhesives & Glue Manufacturer in China
Shenzhen DeepMaterial Technologies Co., Ltd is an innovative company specializing in industrial adhesives for semiconductor,home applicance and electronic applications and surface protection materials for chip packaging and testing. Based on the core technology of adhesives, DeepMaterial has developed industrial adhesives for chip packaging and testing, circuit board level adhesives, PCB conformal coating,PCB potting compound and adhesives for electronic products. Based on adhesives, it has developed protective films, semiconductor fillers, and packaging materials for semiconductor wafer processing and chip packaging and testing.
Guixi Manufacturing Base
Deepmaterial’s industrial park base in Guixi occupies an area of 110 acres, with a construction area of 30,000 square meters in the first phase. There is an A1 adhesive plant and a T1 film manufacturing plant.
Shenzhen Manufacturing Base
Deepmaterial has 1,000 square meters of R&D and sales center and 1,500 square meters of adhesive manufacturing center in Shenzhen.
Standardization Laborator
Shenzhen Protective Film Comprehensive R&D Laboratory 300 Square Meters.Guixi Production Base Analysis And Inspection Laboratory 400 Square Meters.
Best Adhesive Solutions Provider
Strongest Production Capacity
Deepmaterial have two major production bases in Shenzhen and Jiangxi, covering an area of 110 acres, ensure stable supply; Germany produces equipment, Europe and America produces raw materials, and precision testing equipment ensures product stability and quality standards.
Innovative R&D Capabilities
The research and development team led by PhDs in polymer and chemical engineering ensures product development and innovation capabilities. The product has been successfully applied in numerous industries such as aerospace, automotive, medical, photovoltaic, communication, consumer electronics, plastic toys etc.
The Strictest Quality Control
The ISO9001-2015 quality management system and 9 major testing systems ensure stable quality; The product has passed characteristic testing, flame retardant, insulation, waterproof, high and low temperature resistance, and aging resistance level testing.
Adhesive of News and Events
For more news and events, check out our blog.